|
|
 Composition
Composition

VENOSTOR 500 mg Film-coated tablets
Micronized purified flavonoid fraction
1. DENOMINATION
VENOSTOR 500 mg, film-coated tablet.
2. COMPOSITION
p. tablet
Micronized purified flavonoid
fraction.....................................500 mg
. Diosmin (90
%)....................................................................450
mg
. Flavonoids expressed as hesperidin (10
%)......................... 50 mg
Excipients q.s. for one film-coated tablet.
HOW TO TAKE VENOSTOR™ (Diosmin+hesperidin) Tablets
Always take this medicine exactly as described in this manual or
by your doctor or pharmacist. Check with your doctor or
pharmacist if doubt.
The tablets should be taken up at mealtimes. The recommended
dosage is:
Treatment of hemorrhoidal crisis
• 6 tablets per day for 4 days
• then 4 tablets per day for the next 3 days Treatment of
disorders of the venous circulation and DMARD disease
hemorrhoidal:
• 2 tablets per day
Do not take this medication for more than 3 months without
consulting your doctor.
If you have taken more Diosmin+hesperidin that you should If you
have used or taken too Diosmin+hesperidin immediately contact
your doctor pharmacist or poison control center.
GOOD PRACTICES IN HEMORRHOID TREATMENT
Hemorrhoidal
illness represents one of the most common medical conditions in
man. The most recent statistics indicate a prevalence of about
25% in the adult population, and higher than 50% for those older
than 50. However, the prevalence and incidence are most probably
higher, considering the fact that, in certain patients, the
illness begins in an asymptomatic fashion.
The origin of hemorrhoidal disease can be either mechanical or
vascular (hemodynamic):
According to mechanical theory, the supportive structure of the
hemorrhoidal plexus undergoes spontaneous involution which
includes excessive laxity and leads to displacement of the
internal hemorrhoids. Acute intrarectal or abdominal pressure
are worsening factors.
Vascular factors are increasingly recognized as playing an
important role in the development of hemorrhoidal disease
through alterations of the vascular plexuses and dysfunction of
the arteriovenous shunts, the effect of which are amplified, as
above, by increased abdominal pressure.
Hemorrhoidal disease is often associated with inflammation.
Therefore, the treatment of hemorrhoids has to achieve three
objectives: to eliminate mechanical and local triggering
factors, to reduce the inflammation, always present in acute
manifestations and to reestablish optimal hemodynamic and
microcirculatory conditions.
Eliminate mechanical and local
triggering factors
To achieve the first objective it is necessary to observe
certain lifestyle and dietary measures, which represent the
basis of the treatment.
The dietary regime, above all, must include fiber-rich food and
abundant liquids in order to ensure the regularity of the alvus
and to maintain a soft consistency of the stools.
Mechanical laxatives, such as vaseline or liquid paraffin, can
be utilized to this end, as well as avoiding consumption of
stimulating drinks (tea, coffee), alcohol, and spices. Great
care should be paid to personal hygiene and daily habits,
avoiding smoking, a sedentary lifestyle, and a sitting position
for prolonged periods of time.
There is doubt about the role of local treatment, which can be
carried out through agents employed in various combinations:
local anesthetics, anti-inflammatory drugs, lubricating
substances, and substances with local venous tropism.
All these medicines have a favorable effect on clinical
phenomenology, even if, up to now, studies have called into
doubt their real effectiveness.
Reduce the inflammation
The second objective to achieve in the treatment of hemorrhoidal
attacks is prevention of the inflammation. It has been
demonstrated that metabolites of arachidonic acid (in particular
prostaglandin and leukotrienes) levels are higher in the acute
phase.1
Therefore, it is necessary to administer, in acute hemorrhoidal
disease, substances able to antagonize the actions of the
chemical mediators of the inflammation, including the kinins and
the lymphokines, and to inhibit enzymatic activation of
arachidonic acid.
Reestablish optimal hemodynamic and
microcirculatory conditions
Hemodynamic and microcirculatory disorders lead to inflammation
of the tissues and decrease of venous tone. The main
inflammatory mediators involved are PGE-2 and TXA-2, whose
levels are higher during attacks. The clinical manifestations of
these disorders are pain and bleeding, which are very disabling
in the patient’s9 daily
life. The third objective consists therefore in opposing the
PGE-2 and TXA-2 synthesis.
Venostor 500 mg IN THE TREATMENT OF
HEMORRHOIDS
Venostor 500 mg provides complete
efficacy in acute attacks
All symptoms are significantly improved from the second day
of treatment.
A double-blind, placebo-controlled study was performed in
100 patients suffering from hemorrhoidal disease confirmed
by proctoscopy, presenting an acute hemorrhoidal attack of
up to 3 days, which had not been treated. The treatment
lasted 7 days at the dosage of 6
tablets for 4 days and 4 tablets for 3 days.
A clinical examination was performed at D0 and D7, with an
assessment of the symptoms and their improvement using a
4-point scale.
The results of this study demonstrated an overall
improvement of the symptoms significantly higher in patients
treated with Venostor 500 mg in comparison with the controls
(P<0.001), which was already evident on the second day of
treatment, but more marked at the end. The scores used to
evaluate bleeding, pain, and itching resulted in a reduction
in both groups on D7, but to a notably greater degree in
patients treated with Venostor 500 mg (P<0.001); analogous
results were observed regarding tenesmus, with a less
relevant resolution percentage (Figure
1)
and the objective clinical signs (proctitis). Also, the
duration and intensity of the present crisis, compared with
previously, were much more reduced in patients treated with
flavonoids compared with the placebo group (P<0.001) (Figure
2).
Venostor
500 mg seems to offer a comprehensive pharmacological answer to
all the needs of hemorrhoid treatment. Firstly, the flavonoids,
including Venostor 500 mg, have been demonstrated to restain
lysosome enzymes and interfere with enzymes involved in the flow
of arachidonic acid, which causes inflammation.2
Venostor 500 mg has also demonstrated an antioxidant activity,
which allows it to oppose free radicals,3 as
well as a decreasing effect on the synthesis of PGE-2 and TXA-2
by the macrophage.4
All these effects result in a reduction of the pericapillar
permeability,5 and
an increase in the capillary resistance to blood extravasation
in the interstitium.
The hemodynamic effect manifests itself through an increase in
venous tone demonstrated both experimentally6 and clinically.7
Regarding clinical benefits, two recent studies have
demonstrated the outstanding efficacy of Venostor 500 mg, both
in acute and recurrent attacks.
If you forget to take VENOSTOR™ (Diosmin+hesperidin) tablets
Do not take a double dose to make up for the dose that you
missed.
If you stop taking VENOSTOR™ (Diosmin+hesperidin) tablets
Your doctor will tell you how long you need to use VENOSTOR™ (Diosmin+hesperidin)
tablets. Do not stop early treatment.
3. PHARMACEUTICAL FORM
Film-coated tablet.
4. CLINICAL DATA
4.1. INDICATIONS
- Treatment of symptoms related to venolymphatic insufficiency
(heavy legs, pain, early morning restless legs),
- Treatment of functional symptoms related to acute hemorrhoidal
attack.
4.2. DOSAGE AND METHOD OF ADMINISTRATION
- Usual dosage : 2 tablets daily in two divided doses, midday
and evening at meal times.
- Acute hemorrhoidal attack : 6 tablets per day for the first 4
days, then 4 tablets per day for 3 days.
4.3. CONTRA-INDICATIONS
Not applicable
4.4. SPECIAL WARNINGS AND PRECAUTIONS FOR USE
Acute hemorrhoidal attack :
Administration of this medicine is no substitute for the
specific treatment of other anal disorders. The treatment must
be short-term. If the symptoms do not disappear rapidly,
proctological examination should be performed and the treatment
reviewed.VENOSTOR 500 mg 07.2005 2/3
4.5. DRUG INTERACTIONS AND OTHER FORMS OF INTERACTION
Not applicable
4.6. PREGNANCY AND LACTATION
Pregnancy :
Experimental studies in animals have not demonstrated any
teratogenic effect in animals. Furthermore, no adverse effects
have been reported to date in humans.
Lactation :
In the absence of data concerning excretion into breast milk,
breast feeding is not recommended during treatment.
4.7. EFFECTS ON THE ABILITY TO DRIVE AND USE MACHINES
Not applicable
4.8. SIDE-EFFECTS
A few cases of minor gastrointestinal and neurovegetative
disorders have been reported which did not require suspension of
treatment.
4.9. OVERDOSAGE
Not applicable
5. PHARMACODYNAMIC PROPERTIES
5.1. PHARMACODYNAMIC PROPERTIES
Venotonic and vascular protector.
- Pharmacology
It is active upon the return vascular system in the following
way :
. it reduces venous distensibility and stasis,
. in the microcirculation, it normalises capillary permeability
and increases capillary resistance.
- Clinical pharmacology
Double blind controlled studies using methods by which the
effects of the product on venous haemodynamics could be
demonstrated and quantifed have confirmed the above
pharmacological properties in man.
. Dose-effect relationship : a statistically significant
dose-effect relationship was established with respect to venous
plethysmographic parameters : capacitance, distensibility and
rate of emptying. The optimum dose-effect ratio was obtained
with 2 tablets.VENOSTOR 500 mg 07.2005 3/3
. Venous tonic activity : VENOSTOR 500 mg increases venous tone
: venous occlusion plethysmography with a mercury stress gauge
demonstrated a decrease in the rate of emptying.
. Microcirculatory activity : double-blind controlled studies
showed a statistically significant difference between placebo
and the drug. In patients presenting with signs of capillary
fragility, VENOSTOR 500 mg increases capillary resistance, as
measured by angiosterrometry.
- Clinical trials
Double-blind placebo-controlled trials have demonstrated the
activity of the drug in phlebology, in the treatment of chronic
venous insufficiency of the lower limbs (both functional and
organic).
5.2. PHARMACOKINETICS PROPERTIES
In man, following oral administration of the substance
containing 14C Diosmin :
- excretion is mainly faecal, a mean of 14% of the dose
administered is excreted in the urine
- the elimination half-life is 11 hours.
- the drug is extensively metabolised as evidenced by the
presence of various phenol acids in the urine.
5.3.PRECLINICAL SAFETY DATA
Not applicable.
The place of Venostor 500 mg in recent guidelines on the
management of chronic venous disease
by F. Pitsch,France
FURTHER INFORMATION
• The active substance is a flavonoid fraction purified,
micronized (500 mg) comprising 450 mg of Diosmin and 50 mg of
expressed as hesperidin VENOSTOR™.
The other ingredients are: Sodium carboxymethyl starch,
microcrystalline cellulose, gelatin, magnesium stearate, talc;
titanium dioxide (E 171), glycerol, hypromellose, sodium lauryl
sulfate, macrogol 6000, iron oxide yellow (E172), red iron oxide
(E 172).
Aspect of VENOSTOR™ and contents of the pack
The tablets are film-coated, oval and salmon color.
Pack sizes:
Blister pack: 10 tablets / 30 tablet bottle
Box pack: 30 tablets
Chronic venous disease (CVD)
is a common condition representing a spectrum of disorders. Much
effort has been spent creating a common language, which is
essential for the establishment of clinical practice guidelines.
In addition to improvedmethods of defining CVD, there is now
also increased understanding of the pathological processes
involved in its development. Lack of venous tone, abnormal
capillary permeability, and overloaded lymphatic vessels have
been put forward as the mechanisms involved in the development
of CVD. The leukocyte-endothelium interaction and its
association with valvular damage is one of the earliest
pathophysiological mechanisms at work in the disease. This has
focused attention on Venostor 500 mg, the only available
molecule whose activity is known to modify such inflammatory
events. Besides its ability to increase venous tone, regulate
capillary filtration, and speed up lymphatic drainage, it has
been shown to reduce the interaction of leukocytes with the
endothelium in acute venous hypertension and inflammation, and
it is used clinically to treat CVD. Venostor 500 mg has been
intensively investigated in well-designed clinical trials and is
well tolerated. Micronization of the particle size of its
components to <2 μm improves its oral absorption and
bioavailability compared with those of nonmicronized diosmins.
These characteristics explain why Venostor 500 mg is listed
among the venoactive drugs in recent guidelines on the
management of this disease.
Medicographia. 2011;33:306-314 (see French abstract on page 314)
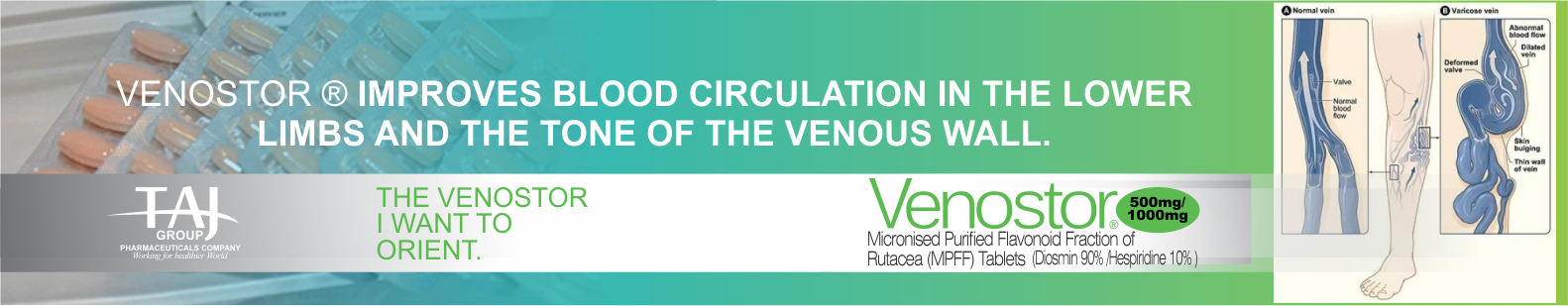
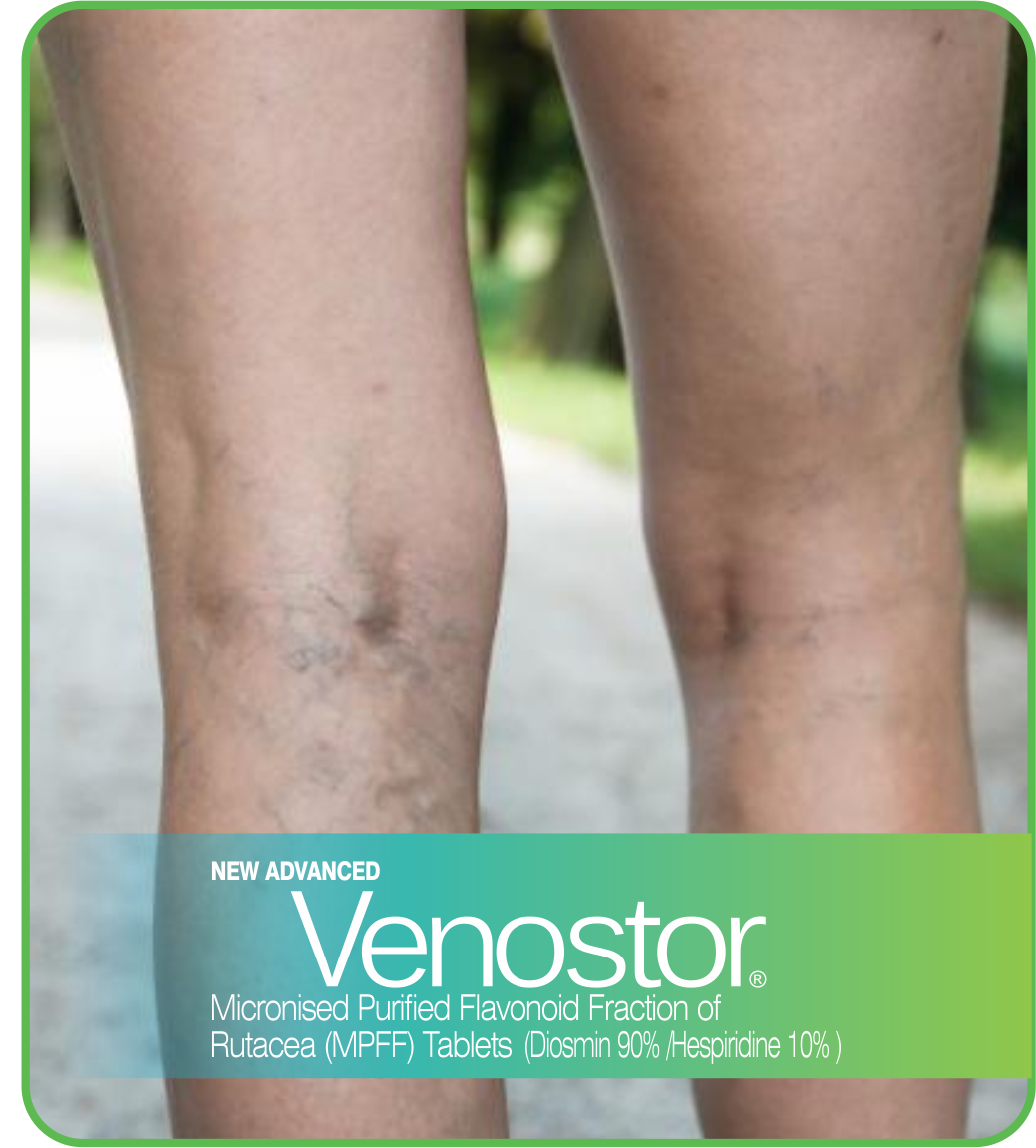
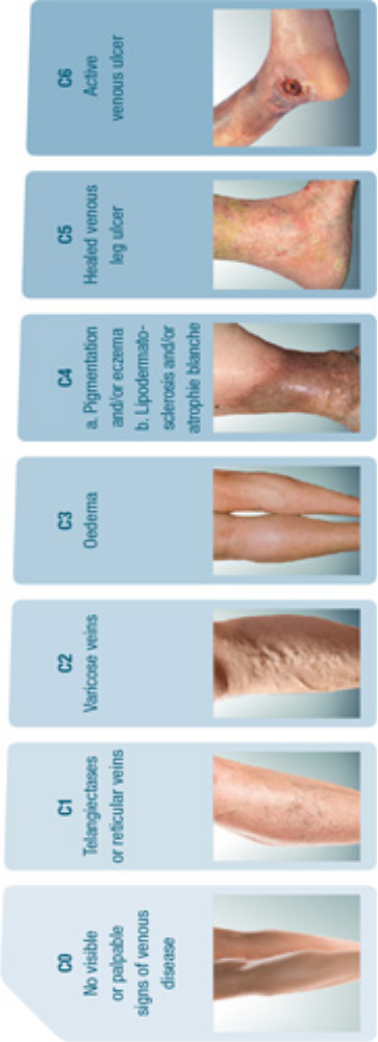
Chronic venous disease (CVD) covers a full spectrum of venous
conditions from telangiectasias to the ultimate complication of
CVD, venous ulcers. Symptoms are commonly associated with signs
of CVD. Venous symptoms are defined as tingling, aching,
burning, pain, muscle cramps, swelling, sensations of throbbing
or heaviness, itching skin, restless legs, and leg tiredness
and/or fatigue, which may be exacerbated by heat or during the
course of a day, and relieved by leg rest, elevation, or both.1
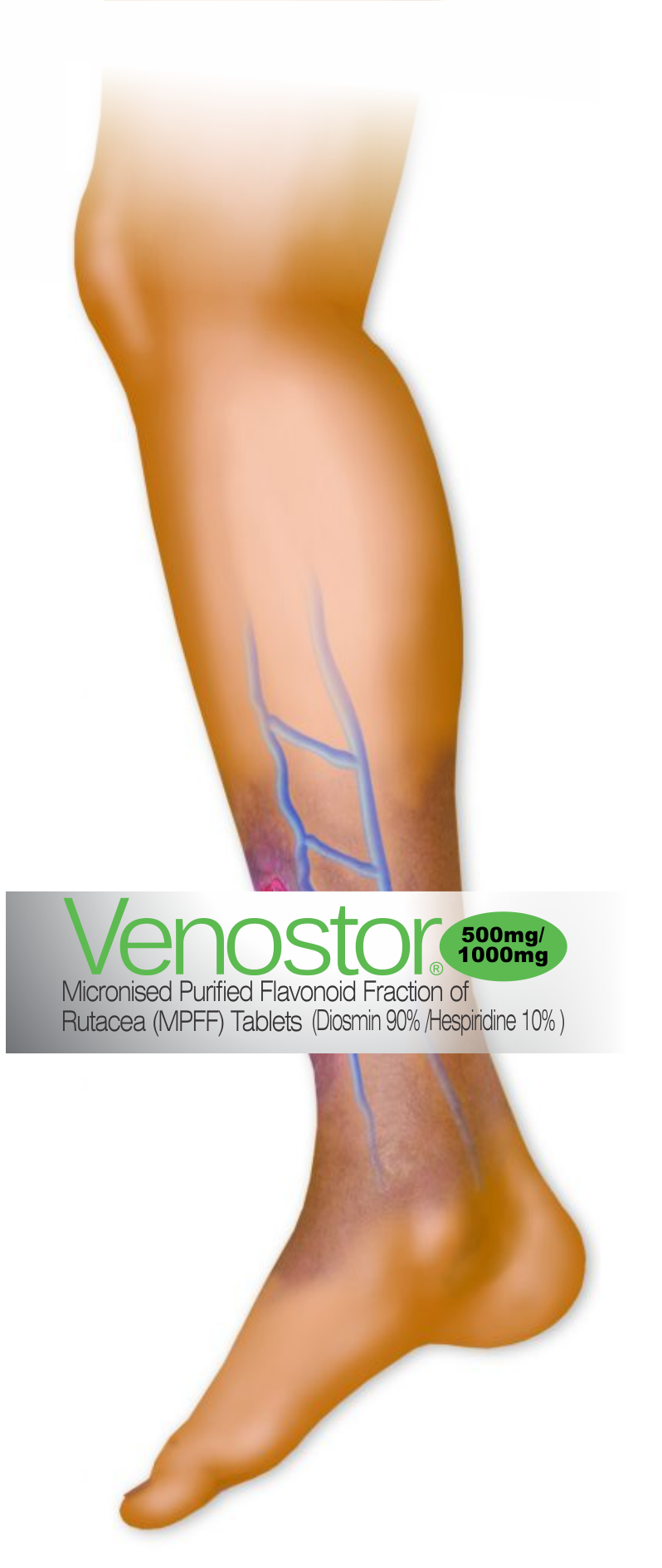
Venous signs are visible manifestations of CVD, which include
dilated veins (telangiectasias, reticular veins, varicose
veins), leg edema, skin changes, and ulcers, as described in the
CEAP (Clinical-Etiological-Anatomical- Pathophysiological)
classification.2,3
This article addresses some of the newer guidelines on
venoactive drugs (VADs) in general, and Venostor 500 mg in
particular, in the management of CVD to help clinicians better
manage patients with CVD of the lower extremity. Intentionally,
only primary CVD will be tackled in this review, putting
postthrombotic venous disease aside.
What are the indications of Venostor 500 mg?
Venostor 500 mg, micronized purified flavonoid fraction (MPFF),
consists of 90% diosmin and 10% other flavonoids (hesperidin,
diosmetin, linarin, and isorhoifolin). Prescribing information
may differ between countries, but in general Venostor 500 mg,
which is available in more than 100 countries, is indicated as a
first-line treatment of symptoms associated with any stage of
CVD, and in lower limb edema. In more advanced disease stages,
such as venous leg ulcer, Venostor 500 mg may be used in
conjunction with sclerotherapy, surgery, and/or compression
therapy, or as an alternative treatment when surgery is not
indicated or is unfeasible.4
Pathophysiology of primary CVD and targets for Venostor 500 mg
treatment
Because they provide a rational explanation for the clinical
benefits of treatments, it is important to consider the
pathophysiological mechanisms underlying any disease in
guidelines. Ambulatory venous hypertension is the hemodynamic
pathology related to all symptoms and signs of CVD. The
underlying components of venous hypertension are failure of the
calf muscle pump, venous valvular incompetence, and luminal
obstruction.5
After prolonged standing, venous pressure in the foot is
approximately 90 mm Hg in both a patient with incompetent venous
valves and a person with a normal leg. However, ambulatory
venous pressures in CVD patients remain high in the lower limbs
during walking (more than 40 mm Hg), whereas normally these
pressures should fall to a lower level (to 30 mm Hg). Due to
valve incompetence, venous refill time on air plethysmography is
shorter in CVD patients compared with healthy individuals.5
When venous pressures in the leg are at higher-than-normal
levels and remain elevated for prolonged periods, a progressive
increase in skin damage may occur. Nicolaides reported that
nearly all patients with exercising venous pressures of >90 mm
Hg experienced venous ulceration.6 How the apparently simple
concept of venous hypertension is responsible for CVD lies in
the complex cellular and molecular processes set in motion by
the abnormal venous hemodynamics it engenders.
CONCLUSION
Hemorrhoids are very
disabling for the patients, and recurrent attacks are frequent.
The inflammation associated with acute crisis, involving
enzymatic activity, free radicals, and inflammation mediators
lead to pain and bleeding. Thanks to its unique pharmacological
properties, Daflon 500 mg meets all the needs of an efficient
hemorrhoidal treatment. The clinical benefits of such properties
are now well documented.
These two studies have demonstrated that the unique micronized
form and comprehensive mode of action of Daflon 500 mg provide
doctors and patients with a faster and stronger efficacy on
acute attacks, at the dosage of 6 tablets for the first 4 days,
and 4 tablets for the next 3 days. This efficacy allows a lower
rate of recurrences, in the long term, and a decreased intensity
and duration of the recurrent attacks (dosage of 2 tablets per
day).
REFERENCES
1. Limasset B, Michel F, Rey R, Guarrigues G, Crastes De Paulet
A. Inflammatory factors in the hemorrhoidal plexus. Proceedings
of European Congress of the International Union of Phlebology.
Budapest. 6-10 Sept 1993.
2. Baumann J, Bruchhausen F, Wurm G. Flavonoids and related
compounds as inhibitors of arachidonic acid peroxidation.
Prostaglandins. 1980;20:627-639.
3. Labrid C. Flavonoids, inflammatory phenomena, and
permeability of capillary vessels. Medicographia. 1989;11:32-39.
4. Damon M, Flandre O, Michel F, Crastes de Paulet A. Effects of
chronic treatment with a purified flavonoid fraction on
inflammatory granuloma in the rat. Arzneimittelforschung/Drug
Res. 1978;37:1149-1153.
5. Galley P. Etude de l’activité de Daflon 500 mg sur la
résistance capillaire. J Int Med. 1987;88:25-26.
6. Labrid C, Duhault J, Vix C. Propriétés pharmacologiques de
Daflon 500. J Int Med. 1987;85:30-36.
7. Cospite M, Milio G, Amato C, Scrivano V, Ferrara F.
Modificazioni dell’emodinamica venosa trattati con alte dosi di
diosminaflavonoidi. Farmaci. 1985;11:451-546.
8. Cospite M. Double blind placebo controlled evaluation of
clinical activity and safety of Daflon 500 mg in the treatment
of acute hemorrhoids. Angiology. 1994;45:566-573.
9. Garner RC, Garner JV, Gregory S, Whattam M, Calam A, Leong D.
Comparison of the absorption of micronized (Daflon 500 mg) and
nonmicronized 14Cdiosmin tablets after oral administration to
healthy volunteers by accelerator mass spectrometry and liquid
scintillation counting. J Pharm Sci. 2002;91:32-40.
|
|